To respond to the challenges faced by companies in the medical devices industry, KEONYS offers a unique solution to improve product development and comply with regulations as rapidly as possible.
Did you know that only 26%* of projects are completed on time, to budget and with the specified functions?
Combined with a highly demanding national and international standards environment, the medical industry, and the medical devices industry in particular, is currently one of the most technically challenging and stimulating fields there is.
This is why KEONYS supports companies in the industry to introduce methods and tools that enable them to improve their product development (design, tests, and processes) while complying with regulations as quickly as possible.
Our service combines a software solution and methodology to address the three following areas:
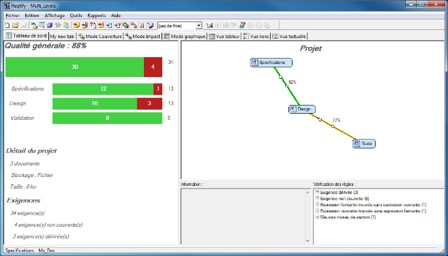
1- Improvement of requirements management in order to deploy on international markets.
At a time when the number of requirements to be met is growing exponentially, as well as time spent on certification applications, KEONYS can provide two kinds of approaches:
- Either the setting up of a simple solution for the traceability of requirements throughout the whole product development cycle;
- Or the setting up of a requirement engineering process, with a full set of tools and a step-by-step approach.
2- The use of simulation to reduce product development and testing time
Patients’ expectations are constantly growing, which means that manufacturers need to innovate through major technological developments.
With this in mind, KEONYS provides a range of simulation tools making it possible to shorten product development cycles, while maintaining a high level of quality. Various types of simulation are available for these applications:
- Simulation and testing of medical device control functions;
- Simulation of products’ physical behavior for approval before the creation of actual prototypes.
3- Improvement of design process management to increase quality and comply with medical industry standards
KEONYS provides a collaborative product lifecycle development and management tool for companies to optimize the management of their processes, technical data, standards and regulations during the design phase. And staying in an unique and innovative environment!
KEONYS also provides comprehensive, step-by-step support to configure and install the solution, based on extensive experience in the deployment of this type of tool.
Contact us for further details regarding this approach and the various topics covered.